TECHNOLOGY
NT2
(Nutrient Transport Technology™ )
Foul-taste isolation, triggered & sustained release, solubility/ bioavailability enhancing, micro-encapsulation technology
- Notoriously foul taste at negligible dosing
- Compromised bioavailability
- Spike & crash phenomena
- Gastrointestinal side-effects
- Negative impact on texture and shelf-life
- Mismatch with manufacturing and logistics SOPs
NT2 - sub 250μ micro-encapsulated powder, optimized for delivery within food and beverage products
An innovative, multi-layered micronized particle, enabling the delivery of sophisticated pharma-class properties in food and beverage products:
ATTRIBUTES
-
Optimal dispersion in liquid formulations
- Hydrophilic(“water-loving”) microencapsulation shell facilitate homogeneous dispersion in beverage and liquid formulations
-
Microencapsulated particles under 250 microns
- Particle size below the sensory threshold of the human mouth
- Inner shell layer controls sophisticated sustained-release profiles
- Outer shell layer controls release triggerss
-
Enhanced water solubility
- Enhancing the solubility of low-bioavailability natural actives
-
Array of sustained-release profiles
- From burst (1h release) to prolonged sustained release (12h)
- In a single formulation, providing each natural active a different release profile, based on its pharmacokinetics
-
Delivery in high water-content food & beverage products
- Lag-time after reconstruction ensures delayed actives’ release, only after consumption
BENEFITS
-
High-dose formulations (15 – 60 pills equivalent)
- Supporting high and effective dosing, up to X30 from current limitations
- High dosing does not compromise positive consumption
-
Complete foul-taste isolation
- A new era of effective microencapsulating, replacing ineffective taste masking
-
Standardizing low-quality botanicals
- Converting low-quality challenging botanical extracts to consistently-performing bioactives with an array of potential pharma-class properties
-
Stability during manufacturing and logistics SOPs
- No modifications required to standard manufacturing SOPs
- No special requirements during logistic transport
-
Consumer-recognized Impact
- Nootropic (brain-impacting) actives enable 15 – 30 minutes to consumer-sensed and recognized impact
- The impact of adaptogens can span between a few hours and a few days, typically depending on the required bioactive “loading” period only after consumption
Final particles in a 250 microns scale-factor

- Bioavailability enhancing matrix
- Sustained-release control
- Water resistance & disintegration trigger control
MAIN CAPABILITIES
CONTROLLED & SUSTAINED RELEASE
Condensing pharma-class triggers & sustained-release properties into sub 250μ particles

ISOLATE
Multiple shell layers resistant to water, isolating the foul taste of natural actives even at high dosing levels

OPTIMAL DISPERSIBILITY
Sophisticated outer shell layer supporting homogeneous “free-float” distribution in liquid formulations
LONG-TERM STABILITY
Microcapsules containing preventive actives prevent release in food & beverage products until encountering GI tract conditions
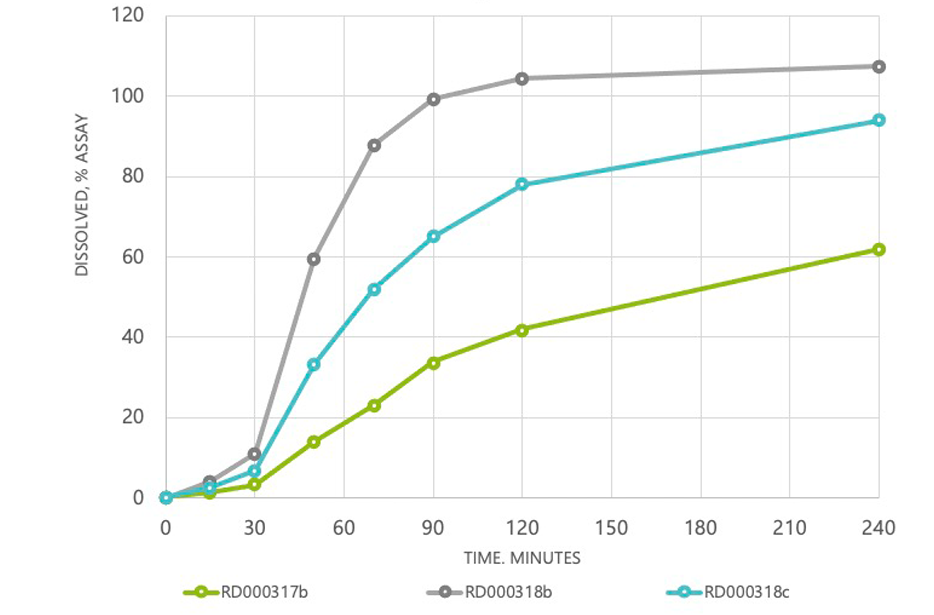
NT2 microencapsulated controlled and sustained release
Example- Extending the caffeine impact for up to 12 hours
15M LAG-TIME; FULL RELEASE IN 60M (1 HOUR)
30M LAG-TIME; FULL RELEASE IN 240M (4 HOURS)
45M LAG-TIME; FULL RELEASE IN 600M (10 HOURS)
Certifications





